Authors' Guidelines
General information
The Journal of Community Health Research (JCHR) is an open access, free of APC charge and peer-review journal that publishes Original Papers, Review Articles, Short Communications, Case Reports, and Letters to the Editor related to all areas of biomedical sciences by an exclusive focus on disease prevention and community health promotion. The journal welcomes all researchers working in different fields of health.
Aims and scope
JCHR is a medical journal (since 2012), which considers Original Papers, Review Articles, Short Communications, Case Reports, and Letters to the Editor in the fields of biomedical science, community-related health issues, epidemiology, health services and economics, primary care, disease prevention, medical education, health promotion, environmental and occupational health, and any fields that take into account delivery of healthcare in the community. JCHR welcomes all research types including study protocols, meta-analyses, specialist studies, and studies that report negative or conflicting results. Therefore, all well-conducted studies are supposed to be published in our journal after a rigorous and transparent peer review process, which may last 3-4 weeks.
We should also note that all our publication procedures are fully open-access and free-access.
Publication charges
The Journal of Community Health Research (JCHR) is an open access journal, and articles processing (AP) is free of charge. Manuscripts should be submitted through the Online Submission System.
Conflict of interest
Editors
This requires all authors to disclose all their financial and personnel relationship that might bias their work. If an organization encompasses any financial interest in the study outcome, it should be mentioned in this part. The possible sources of conflict of interest include having patent or stock ownership, being a member of a company board of directors, or receiving the speaker's fees from a company. In the case that the authors have no conflict of interest, they should state it clearly in this part of the manuscript.
Authors are appealed to provide a declaration that take complete responsibility for the integrity and accuracy of the data. A cover letter should be provided by the corresponding author to list the mentioned items (if applicable) to Editor-in-Chief.
Reviewers
The following situations are considered conflicts and should be avoided:
- Co-authoring publications with at least one of the authors in the past 3 years
- Being colleagues within the same section/department or similar organisational unit in the past 3 years
- Supervising/having supervised the doctoral work of the author(s) or being supervised/having been supervised by the author(s)
- Receiving professional or personal benefit resulting from the review
- Having a personal relationship (e.g. family, close friend) with the author(s)
- Having a direct or indirect financial interest in the pepar being reviewed
Journal Staff
Editorial staff members who participate in editorial must provide editors with a current description of their relationships and activities (as they might relate to editorial judgments) and recuse themselves from any decisions in which an that poses a potential conflict exists. Editorial staff should not use information obtained through working with manuscripts for personal gain.
Editors should regularly publish their own and their journal's staff disclosure statments.
Research involving human
If human are the participants in the work, the authors should include the ethical approval code by ethical committee from institution/organization and should perform the research in accordance with standards based on Declaration of Helsinki in 1964 and its later amendments ethical standards. For this kind of manuscript, an "Ethical approval" should be included in the text the "Code of ethics" section that the authors should declare that the study was performed in accordance with Helsinki declaration. This journal has right to request the related documents from the authors.
Research involving animals
If animals are the goal group of the work, the authors should indicate the international and national guidelines for use of animals. Also, the study should be approved by a research committee at the organization/institution. For this kind of manuscript, an "Ethical approval" should be included in the "Code of ethics" section that the authors should mention the guidelines for the care and use of animals. Also, the ethical code should be added. If the authors do not have results from the studies by any of the authors, they should clear it. This journal has right to request the related documents from the authors.
Informed consent
All research involving human participants or animals must adhere to established ethical standards. For studies involving human subjects, written informed consent must be obtained from all participants prior to their inclusion. Authors must ensure that no identifiable personal information is published. The journal reserves the right to request documentation confirming ethical approval and informed consent procedures.
Ethical considerations:
The Journal of Community Health Research (JCHR), with a rigorous review process and clear ethical policies, supports the publication of high-quality scientific studies. When faced with ethical issues, the journal is committed to investigating and taking the necessary measures to ensure compliance with ethical protocols, the safety of research participants, and adherence to COPE principles.
JCHR follows the Committee on Publication Ethics (COPE)’s flowcharts and guidelines, also “the Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals” issued by the International Committee of Medical Journal Editors (ICMJE) (http://www.icmje.org).
All studies must be approved by the relevant Ethics Committee/ Institution review boards of the respective institutions. As a result, Ethics Code must be mentioned in all studies, except letter to editors, editorials, and review articles. Informed consent forms should be signed by participants . The study itself should follow the ethical standards provided by the Helsinki Declaration, as revised in 2013 available at: www.wma.net/en/30publications/10policies/b3/ .
Ethical considerations in accordance with the ethical standards of the responsible committee on human experimentation must be addressed in the ‘Materials and methods’ or 'Acknowledgment' section. The journal will not consider any manuscript, which is ethically unacceptable. It is the responsibility of the authors/contributors to obtain permissions for reproducing any copyrighted material. Evidence for approval by a local Ethics Committee (for both human as well as animal studies) must be supplied by the authors on demand. The related items should be inserted under the ‘Ethical Considerations’ at the end of the article. A copy of the permission must accompany the manuscript. In the case of not having the Code of Ethics, authors should mention all ethical issues based on the Helsinki Declaration. Conflicts of interest should be declared obviously in each manuscript.
Authors who submit their articles to our journal must:
-
Ensure that their manuscripts are ethically sound and meet the necessary ethical standards reflected in JCHR policies.
-
Present their research findings accurately in the JCHR format including Introduction, Methods, Results, Discussion, and Conclusion.
-
Ensure accurate inclusion of the names of all and only those who qualify for authorship and clearly state their contributions.
-
Disclose any potential competition or conflict of interest at submission.
-
The data and methods used in the research should be presented in sufficient detail in the manuscript so that other researchers can replicate their work. JCHR recommended that the raw data be made publicly available unless there is a compelling reason (eg, participant's confidentiality).
-
Note that submitting the articles to more than one journal at the same time is not ethical and it is considered as misconduct.
-
The results of the research must not previously publish, and any translation must follow our translation policy.
- For any previously published content such as figures or tables, permission to reproduce from the copyright holder is required.
Authorship criteria and contributions:
This Journal also follows the guidelines mentioned in the Recommendations for Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals issued by the International Committee of Medical Journal Editors (ICMJE) (http://www.icmje.org). Each author should meet all the following criteria (1- 4), as recommended by the ICMJE:
1. Substantial contributions in the conception or design of the study or collection, analysis, or interpretation of the data;2. Drafting the work or revising it critically for important intellectual content;
3. Final approval of the version to be published;
4. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
- All communications
- Uploading all submissions
- Revising all drafts of the manuscript
- Submitting a brief description of all contributions to the manuscript in the title page
- Disclosing any information on a prior or duplicate publication or submission of any part of the study elsewhere .
- In this journal, there is no limitation in terms of authorship number for original articles.
Changes in authorship
All authors are required to finalize the order in which the "authors' names" should be listed prior to the first submission. After this step, the journal will not accept any addition or changes. JCHR follows the Committee on Publication Ethics (COPE) policies in this regard.
To request such a change, the editor-in-chief must receive the following from the corresponding author:
- the reason for the change in authors' list and (b) written confirmation (e-mail, letter) from all authors that they agree with the addition, removal, or rearrangement of their names. Furthermore, addition or removal of authors' names should include confirmation from the author who was added or removed. Only in exceptional circumstances will the Editor consider the addition, deletion or rearrangement of authors after the manuscript has been accepted. While the Editor considers the request, publication of the manuscript will be suspended. If the manuscript has already been published in an online issue, any requests approved by the Editor-in-chief will result in a corrigendum.
Editorial policy
We adhere to high standards regarding the editorial policies over publication ethics, scientific misconduct, consent, and peer review criteria.
In this regard, an author is a person who has made substantive contributions in designing the study, analyzing or interpreting the data, drafting or revising the manuscript, as well as verifying all dimensions of the final version of the manuscript with regard to their accuracy or integrity. Researchers who do not meet at least one of the above-mentioned criteria are not considered as an author, but they can be appreciated in the Acknowledgement section.
ORCID
JCHR mandates ORCID iDs for the first and corresponding authors since 2018. Therefore, authors and reviewers are strongly recommended to also connect their Scholar One accounts to ORCID. Read more on the ORCID website.
Copyright
JCHR publishes articles based on the Open Access Agreement.
Upon submitting an article, authors are asked to indicate their agreement with the open access Creative Commons Attribution 4.0 (CC-BY-4.0). Under the terms of this license, authors retain ownership of the copyright of their articles. However, the license permits any user to download, print out, extract, reuse, archive, and distribute the article as long as they provide the appropriate resources along their reports.
Article processing charges
Our journal does not impose any costs on authors for submitting, processing, reviewing, or publishing the manuscript. All the related costs are supplied by Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Scientific misconduct
Scientific misconduct includes fabrication, falsification, and plagiarism with the authors' intention to deceive the publication and/or readers. Honest errors or differences in interpretation are not considered misconduct. Breaches of publication ethics include but are not limited to: failure to reveal financial conflicts of interest; removal of a deserving author or adding a noncontributing author; misrepresenting publication status in the reference list; self-plagiarism without attribution; duplicate or redundant publication; and inclusion of one or more sentences verbatim from another source without citing the original source and putting the sentence(s) in quotation marks.
JCHR takes seriously its responsibility to ensure scientific integrity and will pursue any allegations of misconduct. The JCHR reviewers adhere to the COPE Code of Conduct, which can be found at https://publicationethics.org/membership/code-of-conduct.
Plagiarism, Date Fabrication/Falsification, and Image Mamipulation
Plagiarism is copying text, ideas, images, or data from another source without crediting the original source. If the text is copied from another source, it should be placed between quotation marks and refer to the original source. If the study design is inspired by previous studies, this should be explicitly mentioned.
All manuscripts submitted to JCHR will be checked after submission and before publication in terms of plagiarism using specific software. All submissions will check with iThenticate Professional Plagiarism Prevention software. Submission of a paper implies that it reports unpublished results that are not under consideration for publication elsewhere. If previously published tables, illustrations, or texts are included, this should be clearly indicated in the manuscript and the copyright holder's permission must be obtained. Previously published materials can be cited in a later review or commentary article, but it must be indicated using quotation marks if necessary.
Plagiarism of text from a previously published manuscript by the same or another author is a serious publication offence. Small amounts of text may be used, but only where the source of the quoted material is clearly acknowledged. Reporting fraudulent data or data stolen from other researchers is also unethical and will be treated accordingly. Any alleged offence is considered initially by the Editorial Team.
Once a manuscript is approved to be published in JCHR, the manuscript cannot be published elsewhere (even local journals). The copyright form filled and signed by all authors should be scanned and emailed by the corresponding author to the journal's office (jhr.ssu gmail.com).
gmail.com).
According to JCHR policies, any manipulation of the images in such a way as to lead to a misinterpretation of the information provided by the original image is unethical and not accepted. If image manipulation is detected during the review process or after publication, the paper may be rejected or retracted. We follow COPE policies in this field.
Data fabrication and falsification mean unauthorized creation, presentation, or reporting of research data with the intent of deceiving the academic community are highly unethical and are an example of publication fraud, which is prosecuted in some jurisdictions.
Confidentiality
The Editor(s) and the editorial staff will take all reasonable steps to preserve the confidentiality of the authors 'and reviewers' identities. They must disclose any about a sbmitted manuscript to anyone other than the corresponding author, reviewers, potential reviwers, other editorial advisers, and the publisher, as appropriate.
Guidelines for preparation of manuscript
The manuscript should be submitted online to journal office web site " jhr.ssu.ac.ir" along with the completed copyright form by author(s) and a cover letter to the editor-in-chief via email attachments at jhr.ssu gmail.com (alternative email: jhr
gmail.com (alternative email: jhr ssu.ac.ir).
ssu.ac.ir).
Cover Letter
All authors should sign the cover letter indicating that the manuscripts has not been previously published or submitted for publication in other journals. The cover letter should include the authors' full name, affiliation, academic degree, postal address, telephone, fax number, and email address. Furthermore, conflicts of interest should be declared obviously in the cover letter. Before submission, registration has to be done at the journal's website by the corresponding author.
In the cover letter, all authors must declare their agreement on submitting the manuscript to JCHR. Furthermore, the corresponding author and his/her contact information (address, telephone and fax numbers and e-mail address) should be provided in the cover letter.
Manuscript length
We recommend your article does not exceed 4000 words, with up to five figures and tables. Although this is flexible, exceeding it will impact on the paper’s readability. Authors are suggested to submit figures and images in color. Supplementary and raw data can be published online alongside the article. We also recommend that the Discussion section is no longer than five paragraphs.
Manuscript format
All submitted manuscripts should be in English along with Persian abstracts. Nonnative English speakers may be advised to seek professional help with the language. All materials should be typed with double-line spacing, numbered pages with Times New Roman font (size 11). Abbreviations should be standardized and used just in necessary cases, after complete explanations at the first usage.
Requirements for different types of articles
Original research
The submitted study should have a clear and justified research question.
All articles should include the following parts:
- The article title should convey the study question and design. Titles should not show the study results.
- Abstract (maximum 300 words) should include all the following items. Please note that for RCTs there is a specific CONSORT extension for abstracts):
● Methods: Mention if the study is prospective, randomized, blinded, case control, etc. Mention the number of participating centers. Do not name the center specifically, but provide the geographical location if important. Mention the participants' number, age, gender, and ethnic group. Define the selection, inclusion, and exclusion criteria clearly. Provide information about the interventions, e.g., what, how, when and how long. This part can be removed if there is no intervention.
● Results: Mention the main results with 95% confidence intervals for quantitative studies. Mention the statistical significance level. State absolute rather than relative risks.
● Conclusions: State the major conclusions and their implications. Suggest areas for further research.
●Keywords: Keywords are used for indexing purpose. Each manuscript should provide three to five keywords according to the Medical Subject Headings, MESH (http://www.nlm.nih.gov/mesh/MBrowser.html)
- Introduction: Introduction should provide a background and specify the research objectives. This section must emphasize the rationale for the study. It should neither review the subject extensively, nor include the study results or conclusions
- Methods: This part should include the applied materials and methods clearly. In other words, the the steps taken to acquire the information should be mentioned in details. Repeating details of the standard techniques should be avoided. The software used for statistical analysis should be mentioned. Moreover, the actual methods applied for data collection should be added in details.
- Results: The study findings should be presented in chronological sequence using text, tables, and figures. The results should not be obtained from other studies. Tables and illustrations should be cited in the order that they appear in the text using Arabic numerals.
- Discussion: Discussion should emphasize the new and important aspects of the study. Possible explanations for these findings should be mentioned. Furthermore, the findings can be compared with the similar and conflicting research in the area.
- Conclusion: In this section, overall conclusion of the author(s) from the research, the limitation of the work and the implications of the finding for future research should be noted.
- Acknowledgments: Acknowledgments should be mentioned for any technical help or general, financial, and material support or contributions that need to be acknowledged.
- Conflict of interest: This requires a statement from all authors disclosing all financial and personnel relationship that might bias their work. If an organization encompasses any financial interest in the study outcome, should mentioned here. Authors are appealed to provide a declaration that take complete responsibility for the integrity and accuracy of the data.
-
Funding: Authors are requested to identify financial support, if any, used in the execution of the research and preparation of the manuscript. If there is no funding involved in this study, please specify “This research received no specific financial support from any funding agency.
- Ethical considerations: This section should include information about the independent local, regional or national ethics committee name which approve the proposal, and the ethical consideerations in the research (for example: how to obtain informed consent, confidentiality, etc.). In case of doubt regarding the compliance of the study with the Declaration of Helsinki, the authors should explain the reason for their approach and show that the local, regional and national review committee has clearly approved the questionable aspects of the study. In addition, it is mandatory to send the approval of the ethics committee when submitting the article.
- Code of ethics: This section should be write the code related to article approval.
- Authors’ contribution: This section includes each author’s contribution declared exactly using the authors’ name initials to indicate their identity (for instance, “J.K. and A.D. designed the study; G.H., S.N, and O.W. conducted the experimental work; A.D. analyzed the data; S.N. and O.W. wrote the manuscript, etc.”). In this journal, an author is defined with regard to the recommendations suggested by the International Committee of Medical Journal Editors/ICMJE (http://www.icmje.org). In this regard, all authors should have made significant conceptual, intellectual, experimental, and analytical contributions in conducting, writing, editing, revising, and publishing a study (please see “Authorship criteria and contributions” section in the current instruction).
- Open access policy: "JCHR does not charge readers and their institution for access to its papers. Full text download of all new and archived papers are free of charge."
- References: All manuscripts should be accompanied by relevant references. The Reference list should provide the following information:
Author(s). Title of article. Title of journal. Year; Volume number: Page numbers.
For instance:
- Mahon AK, Haas EJ. A mixed-methods approach to targeting college students’ dairy behaviors. American Journal of Health Behavior.2013; 37(5): 703-10.
- Jafari F, Beladian-Behbahan SE, Samadpour M, et al. Application of the stages of change model to dairy consumption among students of Shahrekord University of Medical Sciences. Journal of Shahrekord University of Medical Science. 2014; 15(6): 65-74. [Persian]
Author(s). Title: sub-title. Edition. Place of publication: Publisher; Year.
For example:
- Miller GD, Jarvis JK, Mc Bean LD. Handbook of dairy foods and nutrition. London: CRC Press; 2007.
Author(s) of chapter. Title: sub-title of chapter. In: Author(s) (or editors) of the book. Title: sub-title of book. Edit no. Place of publication: publisher; Year: page numbers.
For example:
- Stabholz A. Evaluating success and failure. In: Walton RE, Torabinejad M, editors. Principles and practice of endodontics. 3rd ed. Philadelphia: W.B. Saunders Co; 2002:42-45.
Student surname (space) initials. Full title of proposal. [Name of proposal in square bracket]. name of country. name of university, name of faculty; year of publication.
For instance:
- Hadizadeh B. Nickle Ion realeased from as received and recycled NiTi wires in artificial saliva. [Doctorate Thesis]. Iran. Mashhad University of Medical Sciences, Dental School ;2006. [Persian]
Surname of author or authors (space) initials. Paper title. [presentation type (POSTER, LECTURE)] at: number and series of conference; year month day (s) of conference; holder of conference, location of conference, country of conference, city of publisher: publisher name; year of papers publication: first page of paper- last page of paper.
For example:
- Bengtsson S, Solheim BG. Enforcement of data protection, privacy and security in medical informatics. [POSTER] at: Proceedings of the 7th World Congress on Medical Informatics; 1992 Sep 6-10; Geneva, Switzerland. Amsterdam: North-Holland; 1992:1561-1565.
In the case that the references are found in electronic form, use this format:
Surname of author or authors (space) initials. Main title. Available at: URL: full address of website. Access date in order of access by month, day and year.
Note: In the case that the author's name is not included in the website, the title of material should precede.
For example:
-
Carrie A, Bobb R. Mucoceles of the paranasal sinuses. Available at: URL: http://www.bcm.edu/oto/grand/05252000. Accessed April 26, 2008.
Inclusive page numbers should be given for all references. Mention surnames and initials of all authors when there are six or less. In the case of seven or more authors, the names of the first six authors followed by et al. should be listed.
References to papers accepted for publication, which are not published yet, should be cited as in the reference list. The corresponding author is responsible for the accuracy and completeness of the references and for their correct textual citation.
Tables: In limited numbers, tables should be submitted with the captions placed above. Each table should be numbered consecutively with Roman numerals and typed double-spaced, including all headings. Verify tabular statistics to make sure they match the data cited in the text. Do not submit tables as photograph.
Figures: In limited numbers, figures should be provided with high quality art work and mounted on separate pages. The captions should be placed below the figures. The same data should not be presented in tables, figures, and text, simultaneously.
Illustrations: Photographs must be high-contrast, glossy, black and white prints, unmounted and untrimmed, with preferred size of 10 x 15 cm. Color transparencies or photos will be accepted at the discretion of Editorial Board. Written permission must accompany any photograph in which the subject can be identified or any illustration that has been previously published. All illustrations must be numbered as cited in the text in consecutive numeric order.
Abbreviations: Abbreviations that are unavoidable in the abstract must be defined at their first mention there. Moreover, abbreviations should be explained throughout the manuscript.
Clinical Trials
From January 2010, all Clinical Trials must be registered in Iranian Registry of Clinical Trials (www.IRCT.ir) to be considered for publication. This includes all the clinical trials conducted in Iran, even if they are registered in other registration sites. The clinical trials conducted abroad, can be considered for publication in the case that they are registered in a registration site approved by W.H.O.
According to the International Committee of Medical journal Editors (ICMJE), a Clinical Trial is any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions in order to evaluate the effects on intervention on health outcomes.
Review articles
These studies should be prepared according to one of the following styles:
- Systematic reviews: This type of manuscripts should be in the form of meta-analysis, meta-synthesis, or without statistical analysis. These manuscripts contain original articles’ parts. They should contain 2500-4000 words.
- Non-systematic reviews: These kinds of manuscripts should be written by researchers who are expert in the related field. Different parts of such articles include abstract (with no structure), introduction, discussion, and conclusion. They should contain at least 25 references and their number of words should be within the range of 3000-10000 words including the references and captions.
-
Short communication
Sort communications can be in the form of original article, systematic review, or ongoing research, which report interesting findings. The parts in this type of articles are similar to those of original studies, except that ‘Materials and methods’ section should not have any subtitle. These kinds of manuscripts are shorter and prepared in the range of 1500 and 2500 words.
Letter to the editor
Letters to editor are about criticism of previous articles published in this journal or other publications, criticism or review over books, or analysis of a related topic with community health. These manuscripts need no structure and have no abstract. However, their total words' number should not exceed 2000 (maximum) including references. In addition, the references should not exceed more than 10.
Case report
This kind of reseach should include abstract, keywords, introduction, case report, discussion, conclusion, conflicts of interest, acknowledgment, and references. Case reports should have 1500-2500 words. The title should include the words ‘case report’ as well as a description of the reported phenomenon, which indicates a new and emerging community health problem. Three to five key words should be provided.
Editorial
These manuscripts need no structure and have no abstract. The total words' number should not exceed 2500, including references and the references should not exceed 20.
Submission requirements
The authors are required to submit only the final version of the manuscript. The file should be in Microsoft Word using Times New Roman font (size 11). File names must be clearly indicating the contents of each file.
Peer review process:
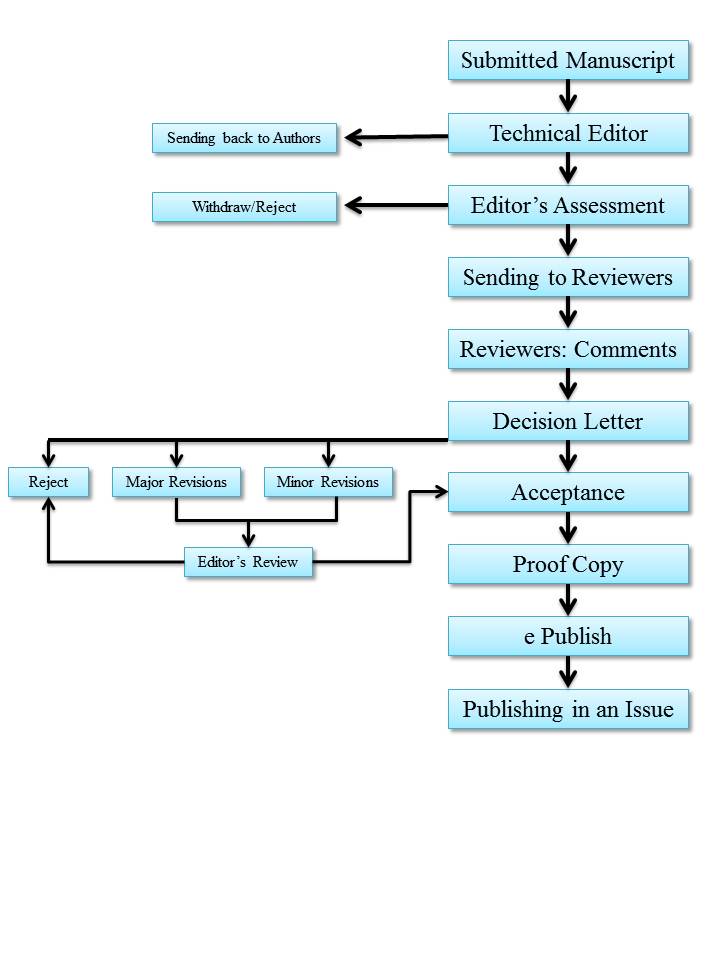
All manuscripts submitted to JCHR will undergo double blind peer-review process. When a manuscript is submitted at this journal website or sent to its E-mail, it will be checked to see if it meets the basic editorial requirements. Later, the manuscript will enter the double blind peer-review process, which will last four weeks in maximum. At this stage, the reviewers check if the study is scientifically credible and ethically appropriate with regard to its scope and methods. They will check if the study was conducted appropriately and analyzed properly. Later, the manuscript will be sent to one in-office referee and two out-of-office ones for the review. Consequently, the corresponding author will be informed about the referee’s remarks: Accepted to publish, Rejected, or Requires revision. Authors can communicate with the editor-in-chief, if they do not wish their manuscript to be reviewed by a particular reviewer due to potential conflicts of interest. The corresponding author can also suggest three expert referees along with their names, affiliation, academic degree, postal address, telephone, and email address. However, the final decision is made by the editor-in-chief to accept the recommendations. It should be noted that the recommended referees should not be from the same institution or university. They also should not be from the same institution or university of the authors.
A manuscript is rejected in the case that at least two reviewers send negative comments about it. The editorial board of the journal, reserves the right to accept or reject the article at any stage and any time or delete a part of the text, tables or figures, if necessary. The editorial board of the journal makes the final decision about each manuscript within a month at maximum and the corresponding author will be informed about the decision immediately.
JCHR considers appeals in the case of rejected manuscripts. In this situation, authors are required to prove that the decision on their manuscript was flawed or not in line with the journal’s policies. To this end, authors should provide a detailed point-by-point response to all the questions raised by reviewers. Authors should not provide a revised manuscript during the appeal since all decisions made on appeals. Please note that: 1) the final decision process on appeals may last longer than the original submissions and 2) appeals must be made within 30 days of the reject decision.
Manuscripts not revised within these time periods are subject to withdrawal from consideration for publication unless the authors can provide extenuating circumstances. The final decision on manuscript's reconsideration will be made by the Editor-in-chief. However, if authors dispute a decision and document good reasons about reconsideration over a manuscript, there will be a rebuttal process. In this case, authors should send their documents to the Editor-in- chief.
- Author(s) must reply to all reviewers' comments in a separate word file, point by point.
- Then this reply file must be uploaded during submitting a revision.
Step II) Reply to comments in Manuscript Word File
- Transfer the comments to only one file.
- After replying the comments, mention that you have done it.
Step III) Highlight Changed Parts in Manuscript Word File
-
Furthermore, author(s) are requested to upload revised word file with highlighted parts. It means that all changes of a word file must be highlighted.
Revision
Manuscripts may be returned to the corresponding author for modification of the scientific content and/or for language revisions. Revised manuscript and a letter containing point-by-point responses to the reviewers' comments must be submitted accompanied by a copy of the original version. Suggestion by the editor about resubmission does not imply that the revised version will be accepted necessarily. If a manuscript, which is returned to the authors for modification, is not resubmitted within two months, it will be regarded as being withdrawn and any revised version received subsequently will be treated as a new manuscript; so, the date of receipt will be altered accordingly. Authors who resubmit a manuscript that has previously been rejected must provide the original manuscript and a letter explaining about modifications made in the manuscript in details.
Accepted manuscripts become the property of this journal.
Galley Proofs
A computer printout will be sent to the corresponding author to be checked for only typographical errors and other essential small changes before publication in order to avoid any mistakes. Major alternations to the text cannot be accepted at this stage. Proofs must be returned to the editor within 7 days after receipt.
In case of any problem, be free to contact us or contact information.
Complaints' Procedures
This procedure is about the complaints regarding the policies, procedures, or actions of the JCHR Editorial Board. All complaints are welcomed since we believe they can provide an opportunity and a spur for or journal's improvement. So, we respond quickly, courteously, and constructively to the complaints.
Our definition of a complaint is as follows:
- The complainant expresses his or her expression of unhappiness as a complaint.
- We infer that the complainant is not a simply disagreement with one of our decisions in terms of the journal's common activities. In other words, we should perceive that there has been a failure of process; for example, a long delay, a rude response, or a severe misjudgment.
- The complaint must be about something that is within the responsibility of the JCHR editorial board-content or process.
How to Make a Complaint
-
The best way to contact with us is by email Jhr
 ssu.ac.ir. Complaints should clearly contain the topic, person, or event related to the JCHR.
ssu.ac.ir. Complaints should clearly contain the topic, person, or event related to the JCHR.
- Whenever possible, complaints will be processed by the relevant member of the Editorial Board. If that person cannot deal with the complaint, it will be send to the executive editor.
- Complaints that are beyond the control of the JCHR Editorial Board will be referred to chairman of the JCHR.
- All complaints will be acknowledged within seven working days.
- Ideally, a full response will be made within four weeks. If this is not possible, an interim response will be given within four weeks. Further interim responses will be provided until the complaint is resolved.
- If the complainant is not happy with the resolution, s/he can ask for the complaint to be forwarded to the Ethics Committee at School of Public Health in Shahid Sadoughi University of Medical Sciences.
- If the complainant remains unhappy, complaints should be forwarded to the Ethics Committee, Shahid Sadoughi University of Medical Sciences, whose decision is final.
The articles published by JCHR are archived on our website. Studies published in JCHR are also available at the following indexing databases:
ISC (Islamic World Science Citation Center )
DOAJ (Directory of Open Access Journals)
OAJI (Open Academic Journals Index)
SID (Scientific Information Database)
Google Scholar
MAGIRAN
Index Copernicus
All printed journal issues are archived in the library of school of public health in Shahid Sadoughi University of Medical Sciences.
Advertising policy
Selecting an editor
Before we start the recruitment process for an editor, we think through what we want to achieve: "What is best for the journal, and what is best for the community that journal serves?"
If the journal and its field are expanding, it needs an editor who can manage the growth. If the journal is no longer serving the needs of its community, it requires an editor who can implement and execute change. In both cases, we work to identify somebody who, working with the publisher, is ready and able to help define a vision for the journal and who has the authority within his or her field to execute that vision. Peer reviewers may make a recommendation about an article, but it is the editor who has the ultimate responsibility to make a final decision on whether to accept or reject an article for publication in a journal.


