Volume 13, Issue 1 (1-2024)
JCHR 2024, 13(1): 228-236 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Nhamo L, Maibouge Tanko M S, Makengo Olivier S. Determinants of Hypertensive Disorders in Pregnant Women Attended at Parirenyatwa Group of Hospitals in Zimbabwe from January - December 2022. JCHR 2024; 13 (1) :228-236
URL: http://jhr.ssu.ac.ir/article-1-1024-en.html
URL: http://jhr.ssu.ac.ir/article-1-1024-en.html
1- Department of Biomedical and Laboratory Sciences, College of Health Agriculture and Natural Sciences, Africa University, Mutare, Zimbabwe
2- Department of Biomedical and Laboratory Sciences, College of Health Agriculture and Natural Sciences, Africa University, Mutare, Zimbabwe ,salissoum@africau.edu
2- Department of Biomedical and Laboratory Sciences, College of Health Agriculture and Natural Sciences, Africa University, Mutare, Zimbabwe ,
Full-Text [PDF 287 kb]
(784 Downloads)
| Abstract (HTML) (1445 Views)
Department of Biomedical and Laboratory Sciences, College of Health Agriculture and Natural Sciences, Africa University, Mutare, Zimbabwe
References
1. Kobashi G. Genetic and environmental factors associated with the development of hypertension in pregnancy. Journal of epidemiology. 2006; 16(1): 1-8.
2. Program NHBPE. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. American journal of obstetrics and gynecology. 2000; 183(1): s1-s22.
3. Tranquilli A, Dekker G, Magee L, et al. The classification, diagnosis, and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Elsevier. 2014; 97-104.
4. Wang L, Ye W, Xiong W, et al. Effects of blood pressure level management on maternal and perinatal outcomes in pregnant women with mild to moderate gestational hypertension. Ginekologia Polska. 2020; 91(3): 137-43.
5. Abalos E, Cuesta C, Carroli G, et al. Pre‐eclampsia, eclampsia, and adverse maternal and perinatal outcomes: a secondary analysis of the World Health Organization Multicountry S survey on M maternal and N newborn H health. BJOG: An International Journal of Obstetrics & Gynaecology. 2014; 121: 14-24.
6. Stuart JJ, Tanz LJ, Missmer SA, et al. Hypertensive disorders of pregnancy and maternal cardiovascular disease risk factor development: an observational cohort study. Annals of internal medicine. 2018; 169(4): 224-32.
7. Noubiap JJ, Bigna JJ, Nyaga UF, et al. The burden of hypertensive disorders of pregnancy in Africa: a systematic review and meta‐analysis. The Journal of Clinical Hypertension. 2019; 21(4): 479-88.
8. Benschop L, Duvekot JJ, van Lennep JER. Future risk of cardiovascular disease risk factors and events in women after a hypertensive disorder of pregnancy. Heart. 2019; 105(16): 1273-8.
9. Tachiweyika E, Gombe N, Shambira G, et al. Determinants of perinatal mortality in Marondera district, Mashonaland East Province of Zimbabwe, 2009: a case-control study. The Pan African Medical Journal. 2011; 8.
10. Muti M, Tshimanga M, Notion GT, et al. Prevalence of pregnancy-induced hypertension and pregnancy outcomes among women seeking maternity services in Harare, Zimbabwe. BMC cardiovascular disorders. 2015; 15: 1-8.
11. Gemechu KS, Assefa N, Mengistie B. Prevalence of hypertensive disorders of pregnancy and pregnancy outcomes in Sub-Saharan Africa: A systematic review and meta-analysis. Womens Health (Lond). 2020; 16: 1745506520973105.
12. Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. The Lancet Global Health. 2014; 2(6): e323-e33.
13. Noubiap JJ, Bigna JJ, Nyaga UF, et al. The burden of hypertensive disorders of pregnancy in Africa: A systematic review and meta-analysis. J Clin Hypertens (Greenwich). 2019; 21(4): 479-88.
14. Raj K, Paul A, Bansal K, et al. Incidence of gestational hypertension among pregnant women in the rural population of District Amritsar-A community-based study. EXECUTIVE EDITOR. 2018; 9(8): 42.
15. Ye C, Ruan Y, Zou L, et al. The 2011 Survey on Hypertensive Disorders of Pregnancy (HDP) in China: Prevalence, Risk Factors, Complications, Pregnancy and Perinatal Outcomes. PLoS ONE. 2014; 9(6).
16. Berhe AK, Kassa GM, Fekadu GA, et al. Prevalence of hypertensive disorders of pregnancy in Ethiopia: a systemic review and meta-analysis. BMC Pregnancy Childbirth. 2018; 18(1): 34.
17. Umesawa M, Kobashi G. Epidemiology of hypertensive disorders in pregnancy: prevalence, risk factors, predictors and prognosis. Hypertens Res. 2017; 40(3): 213-20.
18. Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013; 209(6): 544.e1-.e12.
19. Sengodan SS, Sreeprathi N. Prevalence of hypertensive disorders of pregnancy and its maternal outcome in a tertiary care hospital, Salem, Tamil Nadu, India. International Journal of reproduction, contraception, Obstetrics and Gynecology. 2020; 9(1): 236-40.
20. Larson E, Rabkin M, Mbaruku GM, et al. Missed opportunities to improve the health of postpartum women: high rates of untreated hypertension in rural Tanzania. Maternal and child health journal. 2017; 21: 407-13.
21. Olié V, Moutengou E, Grave C, et al. Prevalence of hypertensive disorders during pregnancy in France (2010-2018): The Nationwide CONCEPTION Study. J Clin Hypertens (Greenwich). 2021; 23(7): 1344-53.
Full-Text: (301 Views)
| Determinants of Hypertensive Disorders in Pregnant Women Attended at Parirenyatwa Group of Hospitals in Zimbabwe from January - December 2022 |
Department of Biomedical and Laboratory Sciences, College of Health Agriculture and Natural Sciences, Africa University, Mutare, Zimbabwe
| ARTICLE INFO | ABSTRACT | |
| Original Article Received: 21 Aug 2024 Accepted: 18 Oct 2024 |
Background: Globally 2.73% of pregnant women suffer from hypertension. Currently, there is still a gap of knowledge in Zimbabwe on hypertensive disorders of pregnancy hence the present study. Methods: An analytical cross-sectional study was conducted. The structured checklist was used to extract data. HBP was measured two times on the right arm using an electronic device (OMRON HEM-7261) with an average of two readings. Patient records were used to collect secondary data from prenatal care visits and laboratory tests. We used simple random sampling methods to screen prenatal care records for pregnant women. All pregnant women at Parirenyatwa Group of Hospitals in 2022 were eligible and constituted our study population. The sample size was determined statistically by power analysis using epidemiological information, this yielded a sample of 240. The Prevalence of HBP in pregnancy and risk factors were determined by Fisher exact test using Graph Pad (Prism version6.0), P < 0.05 was set as statistically significant. Results: 240 pregnant women were enrolled given a prevalence of HBP of 25%. Most pregnant women having HBP are 36 to 40 years old and located in urban areas (92%, n = 55). Gestational age 33 % (n = 5), Gravida 3-4 (33%, n = 13) Parity 3-4 (74%, n = 25), and Multiple Pregnancy (25%, n = 5) were the most associated with HBP. Pre-existing hypertension was the most common comorbidity 92%.Family history of HBP (OR = 4.62, 95%, CI = 1.4-15.1), p = 0.016) and age being < 30 years OR= 0.26, 95%, CI = 0.14-0.49), p = 0.0001) were associated with HBP. Conclusions: The prevalence of HBP in pregnancy was higher (25%) than the worldwide prevalence of 2.7% and risk factors are mainly age and family history of HBP. Keywords: Prevalence, High blood pressure, Determinants, Pregnancy |
|
 |
||
Corresponding Author: Mahamane Salissou Maibouge Tanko
salissoum@africau.edu |
How to cite this paper:
Nhamo L, Salissou Maibouge Tanko M, Makengo Olivier S. Determinants of hypertensive disorders in pregnant women attended at Parirenyatwa Group of Hospitals in Zimbabwe from January - December 2022. J Community Health Research 2024; 13(1): 228-236.
Introduction
Nhamo L, Salissou Maibouge Tanko M, Makengo Olivier S. Determinants of hypertensive disorders in pregnant women attended at Parirenyatwa Group of Hospitals in Zimbabwe from January - December 2022. J Community Health Research 2024; 13(1): 228-236.
Introduction
Hypertensive disorders of pregnancy (HDP ) is a spectrum of conditions of vascular origin and systemic manifestations having mixt etiology of genetic and acquired factors taking place during pregnancy Women can develop different types of high blood pressure during pregnancies which are chronic hypertension, gestational hypertension, or preeclampsia This commonly used classification takes into account the time of appearance of the condition about pregnancy (1, 2, 3). In addition Stroke, placental abruption, preterm deliveries, intra-uterine growth retardation (IUGR), intra-uterine death, as well as maternal morbidity and mortality are some of the complications experienced with pregnancy (4). Worldwide 2.73 % of pregnant women suffer from hypertension. Incidence of chronic hypertension, preeclampsia, and eclampsia have been recorded to be 0.29%, 2.16%, and 0.28% in that order (5). High blood pressure during pregnancy has been linked to risk factors such as type 2 diabetes, chronic hypertension, and raised blood lipids associated with rapid urbanization and changing lifestyles (6). While blood pressure measurement is routinely monitored as a part of antenatal care, it is important to get a better understanding of the burden of hypertension during pregnancy and its associated risk factors. Identification of modifiable risk factors would be vital for the primary prevention of this condition and for avoiding adverse maternal and fetal outcomes.
Nevertheless, in Africa, the prevalence of hypertension in pregnant women is higher (7). In Harare, Zimbabwe an increase in referrals due to high blood pressure in pregnant women to central hospitals from 2009 to 2011 has been experienced. High blood pressure in the past years till date has caused complications during and after pregnancy (8). Maternal and perinatal mortality in Zimbabwe study done by Tshimamga et al in 2009 proved that high blood pressure in pregnant women was one of the top five causes of maternal mortality and the third highest reason for referral in labor (9). Mortality of mothers giving birth has been increasing in the past years at Parirenyatwa Group of Hospitals because of high blood pressure in pregnant women. An increase in referrals for gestational hypertension from 20.7% in 2009 to 44% in 2011 has been recorded in central hospitals. The studies done before did not outline if this was a prevalence or case management at the primary care level (10). In addition, the overall and type-specific prevalence of hypertensive disorders of pregnancy (HDP) and associated risk factors are unknown in Sub-Saharan Africa including Zimbabwe (11). Hypertensive disorders of pregnancy contribute to 14% and 16% of maternal mortality in the world and in Sub-Saharan Africa, respectively (12). Hence there is a need to determine prevalence based on region, the subtype of HDP, and risk factor, this will educate policymakers and guide strategies for early detection, prevention, and management of these disorders in Zimbabwe. Currently, Harare City has experienced an increase in referrals due to Hypertensive disorders of pregnancy to Parirenyatwa central hospitals, Despite this increase the prevalence of subtypes of HDP and their associated risk factor are currently unknown The aim of the current study was to provide recent data to add on body of knowledge on the prevalence and risk factors of high blood pressure in pregnant women at Parirenyatwa Hospital for the period of January 2022 - December 2022.
Methods
Study Design, Statistical population, sample, and sampling method
This research was an analytical cross-sectional study conducted at the Mbuya Nehanda maternity ward unit located at Parirenyatwa Hospital, in Zimbabwe. Observations at the hospital were done and data was collected. It described the prevalence rate of high blood pressure in pregnant women and potential risk factors associated. The study included all women who were pregnant with or without high blood pressure at Parirenyatwa Group of Hospitals for the period of January to December 2022 while non-pregnant and women on termination of pregnancy were excluded. To determine the Sampling Size we have used the formula N = z2. [p*q]/d2), where N = sample size zα/2 = related to Normal distribution for alpha equal to 5 % is 1.96, d = absolute precision/level of significance. P = expected prevalence, q = 1-p, using the previous study carried out by Muti et al 2015, the prevalence of pregnant women with high blood was assumed to be 19.4% (10). When the prevalence is between 10-90 %, the precision is recommended to be approximately below 5%. Sample size, N = (1.96/0.05)2 X 0.194 (1-0.194) = 240.
Tools
Patient records were used to collect data. Midwives and gynecologists were also asked questions for the collection of data. Experienced Midwives' and gynecologists recorded Blood pressure. High blood pressure was measured two times on the right arm of the selected subject using an automatic electronic device (OMRON HEM-7261). The average of two readings was used. The patient sat in a comfortable position, remained quiet and the arm cuff was placed on the patient’s arm. The cuff inflated and then slowly deflated and the measurement was read on the machine Simple random sampling was used to sample our sample size of 240 women out of the total study population of all pregnant women with or without HBP admitted at Parirenyatwa hospital during the year 2022.
Data Collection
After obtaining approval from the local ethics committee (AUREC), AUREC Approval letter Number (AUREC 2616/23), and permission from Parirenyatwa Hospital, data was collected from patients’ records. Data from the prenatal care visits and laboratory tests was collected. Permission from the prenatal care supervisor and laboratory manager was required. Data was collected from record review using a structured and pre-tested checklist. The training was given to both data collectors and supervisors. Three midwives were assigned to collect the data; one supervisor was assigned to supervise the quality of data collection.
Data analysis procedures
Records of pregnant women's hypertensive and non-hypertensive cases were collected via their medical records retrieved and checked for hypertension during pregnancy. Records that show one of the four HDP types (gestational hypertension, chronic hypertension, pre-eclampsia/ eclampsia, or superimposed hypertension) and non-hypertensive all were counted under each group. The criterion used was an elevation of blood pressure for gestational hypertension whereas blood pressure, protein urea, and other laboratory investigations were used as criteria for other types of hypertensive disorders. Then finally descriptive and inferential statics were used to determine factors that were associated with hypertension. Descriptive statistics such as frequency, a measure of central tendency, and a measure of dispersion were calculated. Data such as socio-demographic factors including age, blood pressure recordings medical history of the patient. Data was collected from patients records who were between the ages of 20 and 44 years. Other demographic characteristics like religion were also considered so that we were able to know if the causes of high blood pressure are religiously related. Socioeconomic characteristics were also used for data collection so that we know those who have a lower socioeconomic status and cannot afford antenatal care checkups.
Data Analysis
Our dependent variable was Hypertensive disorder of pregnancy. Our Independent variables were Demographic factors such as age, Residential area, marital status, Plan of pregnancy, Previous Obstetric factors: and Gravida Parity. Abortion histories, Antenatal Care (ANC) follow-up, and Multiplicity of pregnancies were also included as well as previous disease conditions: Pre-existing hypertension and family history of hypertension. For risk factors analysis we have conducted randomization to minimize bias and the effect of confounding variables through a random mechanism to select a sample from our target population hence through randomization we ensured that each patient has an equal chance of being enrolled in the study, where we generate comparable comparison groups, which are alike in all the important aspects. Hence the independent variable values were randomized to eliminate the selection bias, and for the groups concerning many known and unknown confounding or prognostic variables the Fisher exact test was conducted CI OR (Odd Ratio), and the P value was set at P < 0.05 as statistically significant for risk factors analysis A descriptive statistic test of frequency was used to establish the prevalence. Graphic Pad (version) statistical software was used to analyze data.
Results
Socio-demographic and clinical characteristics of study participants
Demographic characteristics analysis revealed that the majority of the study participants were from Harare city. The majority of pregnant women with hypertension are in the age range of 36-40 years old and most of them were married and have their pregnancy. Furthermore increase in age is directly proportional to the increased risk of HBP More than half of the participants were Christians and employed, Table 1.
Nevertheless, in Africa, the prevalence of hypertension in pregnant women is higher (7). In Harare, Zimbabwe an increase in referrals due to high blood pressure in pregnant women to central hospitals from 2009 to 2011 has been experienced. High blood pressure in the past years till date has caused complications during and after pregnancy (8). Maternal and perinatal mortality in Zimbabwe study done by Tshimamga et al in 2009 proved that high blood pressure in pregnant women was one of the top five causes of maternal mortality and the third highest reason for referral in labor (9). Mortality of mothers giving birth has been increasing in the past years at Parirenyatwa Group of Hospitals because of high blood pressure in pregnant women. An increase in referrals for gestational hypertension from 20.7% in 2009 to 44% in 2011 has been recorded in central hospitals. The studies done before did not outline if this was a prevalence or case management at the primary care level (10). In addition, the overall and type-specific prevalence of hypertensive disorders of pregnancy (HDP) and associated risk factors are unknown in Sub-Saharan Africa including Zimbabwe (11). Hypertensive disorders of pregnancy contribute to 14% and 16% of maternal mortality in the world and in Sub-Saharan Africa, respectively (12). Hence there is a need to determine prevalence based on region, the subtype of HDP, and risk factor, this will educate policymakers and guide strategies for early detection, prevention, and management of these disorders in Zimbabwe. Currently, Harare City has experienced an increase in referrals due to Hypertensive disorders of pregnancy to Parirenyatwa central hospitals, Despite this increase the prevalence of subtypes of HDP and their associated risk factor are currently unknown The aim of the current study was to provide recent data to add on body of knowledge on the prevalence and risk factors of high blood pressure in pregnant women at Parirenyatwa Hospital for the period of January 2022 - December 2022.
Methods
Study Design, Statistical population, sample, and sampling method
This research was an analytical cross-sectional study conducted at the Mbuya Nehanda maternity ward unit located at Parirenyatwa Hospital, in Zimbabwe. Observations at the hospital were done and data was collected. It described the prevalence rate of high blood pressure in pregnant women and potential risk factors associated. The study included all women who were pregnant with or without high blood pressure at Parirenyatwa Group of Hospitals for the period of January to December 2022 while non-pregnant and women on termination of pregnancy were excluded. To determine the Sampling Size we have used the formula N = z2. [p*q]/d2), where N = sample size zα/2 = related to Normal distribution for alpha equal to 5 % is 1.96, d = absolute precision/level of significance. P = expected prevalence, q = 1-p, using the previous study carried out by Muti et al 2015, the prevalence of pregnant women with high blood was assumed to be 19.4% (10). When the prevalence is between 10-90 %, the precision is recommended to be approximately below 5%. Sample size, N = (1.96/0.05)2 X 0.194 (1-0.194) = 240.
Tools
Patient records were used to collect data. Midwives and gynecologists were also asked questions for the collection of data. Experienced Midwives' and gynecologists recorded Blood pressure. High blood pressure was measured two times on the right arm of the selected subject using an automatic electronic device (OMRON HEM-7261). The average of two readings was used. The patient sat in a comfortable position, remained quiet and the arm cuff was placed on the patient’s arm. The cuff inflated and then slowly deflated and the measurement was read on the machine Simple random sampling was used to sample our sample size of 240 women out of the total study population of all pregnant women with or without HBP admitted at Parirenyatwa hospital during the year 2022.
Data Collection
After obtaining approval from the local ethics committee (AUREC), AUREC Approval letter Number (AUREC 2616/23), and permission from Parirenyatwa Hospital, data was collected from patients’ records. Data from the prenatal care visits and laboratory tests was collected. Permission from the prenatal care supervisor and laboratory manager was required. Data was collected from record review using a structured and pre-tested checklist. The training was given to both data collectors and supervisors. Three midwives were assigned to collect the data; one supervisor was assigned to supervise the quality of data collection.
Data analysis procedures
Records of pregnant women's hypertensive and non-hypertensive cases were collected via their medical records retrieved and checked for hypertension during pregnancy. Records that show one of the four HDP types (gestational hypertension, chronic hypertension, pre-eclampsia/ eclampsia, or superimposed hypertension) and non-hypertensive all were counted under each group. The criterion used was an elevation of blood pressure for gestational hypertension whereas blood pressure, protein urea, and other laboratory investigations were used as criteria for other types of hypertensive disorders. Then finally descriptive and inferential statics were used to determine factors that were associated with hypertension. Descriptive statistics such as frequency, a measure of central tendency, and a measure of dispersion were calculated. Data such as socio-demographic factors including age, blood pressure recordings medical history of the patient. Data was collected from patients records who were between the ages of 20 and 44 years. Other demographic characteristics like religion were also considered so that we were able to know if the causes of high blood pressure are religiously related. Socioeconomic characteristics were also used for data collection so that we know those who have a lower socioeconomic status and cannot afford antenatal care checkups.
Data Analysis
Our dependent variable was Hypertensive disorder of pregnancy. Our Independent variables were Demographic factors such as age, Residential area, marital status, Plan of pregnancy, Previous Obstetric factors: and Gravida Parity. Abortion histories, Antenatal Care (ANC) follow-up, and Multiplicity of pregnancies were also included as well as previous disease conditions: Pre-existing hypertension and family history of hypertension. For risk factors analysis we have conducted randomization to minimize bias and the effect of confounding variables through a random mechanism to select a sample from our target population hence through randomization we ensured that each patient has an equal chance of being enrolled in the study, where we generate comparable comparison groups, which are alike in all the important aspects. Hence the independent variable values were randomized to eliminate the selection bias, and for the groups concerning many known and unknown confounding or prognostic variables the Fisher exact test was conducted CI OR (Odd Ratio), and the P value was set at P < 0.05 as statistically significant for risk factors analysis A descriptive statistic test of frequency was used to establish the prevalence. Graphic Pad (version) statistical software was used to analyze data.
Results
Socio-demographic and clinical characteristics of study participants
Demographic characteristics analysis revealed that the majority of the study participants were from Harare city. The majority of pregnant women with hypertension are in the age range of 36-40 years old and most of them were married and have their pregnancy. Furthermore increase in age is directly proportional to the increased risk of HBP More than half of the participants were Christians and employed, Table 1.
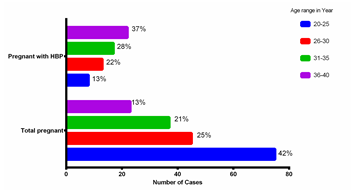
Table 1. Socio-demographic characteristics
| Variables | Total Pregnant N = 180 | Pregnant with HBP N = 60 |
| Age Groups (Years) 20-25 26-30 31-35 36-40 |
75 (42%) 45 (25%) 37 (21%) 23 (13%) |
8 (13%) 13 (22%) 17 (28%) 22 (37%) |
| Urban Residential Area Yes No |
153 (85%) 27 (15%) |
55 (92%) 5 (8%) |
| Marital Status Yes No |
160 (89%) 20 (11%) |
50 (83%) 10 (17%) |
| Plan of Pregnancy Yes No |
130 (72%) 50 (28%) |
40 (67%) 20 (33%) |
| Christianity Yes No |
120 (67%) 60 (33%) |
35 (58%) 25 (42%) |
| Employment Yes No |
100 (56%) 80 (44%) |
40 (67%) 20 (33%) |
Gestational age for the study participants was mostly in the 3rd (67% for pregnant women without hypertension and 33% for pregnant women with hypertension), `32 cases 2nd (78% of pregnant women without HBP and 22% of women with HBP), and lastly 1st trimester (75% pregnant without HBP and 25% for pregnant with HBP). Gravida, parity, multiplicity of pregnancy, and ANC visits were also other obstetric factors that were looked at during the study. Hypertension in pregnancy is high in the late stages of pregnancy (i.e. 3rd trimester -45 patients out of 60), Table 2. Many pregnant women visit the hospital during their 3rd trimester but a few go to ANC visits more than 2 times. Both gravida and parity also increase more at greater than 4.
During the time the study was done a few women had twins no triplets or quadruplets were recorded, Table 2.
During the time the study was done a few women had twins no triplets or quadruplets were recorded, Table 2.
Table 2. Obstetric factors associated with pregnancy at PGH in 2022 (N = 240)
| Obstetric Factor | Pregnant, without HBP N = 180 |
Pregnant with HBP N = 60 |
Total (with + without HBP) |
| Gestational age 1st trimester 2nd trimester 3rd trimester |
10 (67%) 35 (78%) 135 (75%) |
5 (33%) 10 (22%) 45 (25%) |
15 45 180 |
| Gravida 1-2 3-4 > 4 |
53 (76%) 27 (68%) 100 (77%) |
17 (24%) 13 (33%) 30 (23%) |
70 40 130 |
| Parity 1-2 3-4 > 4 |
65 (74%) 25 (74%) 90 (76%) |
23 (26%) 9 (74%) 28 (24%) |
88 34 118 |
| ANC visits 1-2 3-4 > 4 |
95 (76%) 35 (66%) 50 (81%) |
30 (24%) 18 (34%) 12 (19%) |
125 53 62 |
| Multiplicity of pregnancy | 15 (75%) | 5 (25%) | 20 |
Risk factors associated with HBP in pregnancy
The main potential statistically significant risk factors associated with HBP in pregnancy were age < 30 years old OR 0.26 95% CI (0.14-0.49),
P = 0.0001, and family history of HBP
OR 4.62 95% CI (1.4-15.1) P = 0.016 (Fisher exact test)
The main potential statistically significant risk factors associated with HBP in pregnancy were age < 30 years old OR 0.26 95% CI (0.14-0.49),
P = 0.0001, and family history of HBP
OR 4.62 95% CI (1.4-15.1) P = 0.016 (Fisher exact test)
Table 3. Disease factors associated with HBP during pregnancy (Fisher's exact test, N = 240)
| Variable | Pregnant no HBP N = 180 |
Pregnant+ HBP N = 60 |
OR 95% CI |
P Value two-sided |
RR at 95%CI |
| Pre-existing hypertension Yes No |
20 (11%) 160 (89%) |
5 (8%) 55 (92%) |
0.72 (0.26-2.03) | 0.63 | 0.75 (0.29- 1.91) |
| Family History of HBP Yes No |
5 (3%) 175 (97%) |
7 (12%) 53 (88%) |
4.62 (1.4-15.1) | 0.016 | 4.2 (1.38- 12.75) |
| History of DM Yes No |
15 (8%) 155 (92%) |
2 (3%) 58 (97%) |
2.5 (0.5 13.2) | 0.25 | 2.2 (0.5 -8.57) |
| Age (Years) ≤ 30 > 30 |
120 60 |
21 39 |
0.26 (0.14-0.49) | 0.0001 | 0.52 (0.36 0.75) |
O R: Odd Ratio. C I: Confidence Interval, RR: Relative Risk
Prevalence of High Blood Pressure in pregnancy stratified by type of hypertension
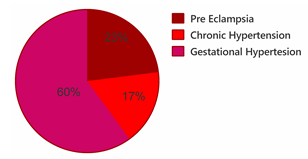
Of the 240 patients who were involved in the study, the majority had gestational hypertension (60%) followed by pre-eclampsia and the least common finding was chronic hypertension (Figure 1).
Of the 240 patients who were involved in the study, the majority had gestational hypertension (60%) followed by pre-eclampsia and the least common finding was chronic hypertension (Figure 1).

Figure 1. Prevalence of disorders of HBP in pregnancy stratified by categories
Prevalence of hypertension in pregnant women stratified by age groups
The majority of the study participants were in the age group of 20-25 years and the last group was in the age range of 36-40 years (12.5%). Patients with hypertension in pregnancy are in their majority found in the 35-40 years age group (37%). Women in the age group of 35-40 years were almost 3 times more likely to suffer from high blood pressure compared to younger women. The total number of patients involved in the study was 240 and those diagnosed with hypertension were 60 patients and this yielded a prevalence of HBP in pregnant women of 25% (Figure2).
The majority of the study participants were in the age group of 20-25 years and the last group was in the age range of 36-40 years (12.5%). Patients with hypertension in pregnancy are in their majority found in the 35-40 years age group (37%). Women in the age group of 35-40 years were almost 3 times more likely to suffer from high blood pressure compared to younger women. The total number of patients involved in the study was 240 and those diagnosed with hypertension were 60 patients and this yielded a prevalence of HBP in pregnant women of 25% (Figure2).

Figure 2. Prevalence of HBP in pregnancy stratified by age group
Discussion
Reports from systematic review and meta-analysis including studies from 24 African countries revealed a significantly higher Prevalence of Hypertensive disorders of pregnancy (HDP) in Central and Western Africa; where the burden of HDP in Africa is high, with about one in 10 pregnancies affected (13). In the case of Zimbabwe, our present study indicated socio-demographic characteristics that in majority of the study participants are from urban areas and the majority are in the age range of 20 to 30 years old. In this study, HBP is more common in Urban than rural and a similar finding was found in a study by Raj et al 2018 where a low prevalence was found in rural settings (14). Our findings indicate high prevalence within the age range of 31 to 40 years similar finding was found in a study conducted by Ye et al 2014 in China (15) and Berhe, A. K et al 2018 in Ethiopia (16). The opposite study by Umesawa et al 2017 found a low prevalence (17) our study found a high prevalence of gestational hypertension and preeclampsia Our finding indicates a high prevalence within the age range of 31 to 40 years as reported previously gestational hypertension and preeclampsia increased with gestational age (18). And in this present study this could be associated with unemployment and interrupted ANC which are the most predominant finding associated with these conditions. Umesawa, et al 2017 study similar to our study finding lower education appears to be a modifiable risk factors for HBP. Furthermore similar to their study, our study indicated preexisting hypertension and diabetes mellitus appear to be non-modifiable risk factors (17). The obstetrical factor associated with HBP in pregnancy in this present study is mainly Gestational age at 3rd trimester of pregnancy, Parity 3-4, ANC 1,2. In our study, the prevalence is low among multigravida (25%) while opposite findings were reported in a study conducted in India where The prevalence was high primigravida (54%) compared to multigravida (46%) (19). As the number of fetuses a mother is carrying increases (multiplicity of pregnancy) the risk of high blood pressure in pregnancy increases. Preventing and/or managing high blood pressure in pregnancy lowers the risk of low birth weight and its implications. Another risk factor for high blood pressure in pregnancy includes the fact that pregnant women are facing delays in being attended to during their ANC visits. This is caused by a lack of staff, ambulances, or other vital resources (these include BP machines), which raises the risk of maternal and newborn mortality due to high blood pressure. The prevalence of high blood pressure in pregnant women was 25% (60/240) which is still high when compared to the worldwide Global prevalence, of 2.7% in pregnant women HDP and low when compared to the Tanzania study which found it to be 26.7% (20). Among potential risk factor that significantly leads to HBP found in this study are mainly the family history of high blood pressure and being aged 30 years old and above and this corroborates with studies conducted by (Umesawa et al 2017, and Valerie et al 2021) which reported similar findings (17, 21). It might also be a result of more pregnancies being scheduled as a result of the drop in maternal costs. Many women tend to get pregnant easily since maternal costs are low now, thus a large number of pregnant women were present in the study. Furthermore, the high prevalence of high blood pressure in pregnant women was linked to certain characteristics such as multiplicity of pregnancy. According to previous studies, chronic hypertension, preeclampsia and have been recorded to be 0.29%, and 2.16% in that order (5). Gestational hypertension was 20.7% in 2009 to 44% in 2011 which is still low when compared to this present study which found prevalence for preeclampsia, chronic hypertension, and gestational hypertension at 23%, 17%, and 60% respectively. This showed a high prevalence of high blood pressure in pregnant women stratified by high blood pressure types. The present study reported a high prevalence of Gestational hypertension and pre-eclampsia when compared to a study conducted in India which reported a low prevalence of Gestational hypertension and pre-eclampsia and this disparity could be likely be attributed to differences in study design and study setting which differ among the two studies. However, Similarities with our findings were reported by Valérie et al 2021 in a study conducted in France where chronic hypertension and pre-eclampsia were associated with HBP during pregnancy (21).
Despite previous study has been done in Zimbabwe on the prevalence of HBP in pregnant women (Muti M, 2015) still there has not been a reduction in these morbidity and mortality rates rather there has been an increase in the rates therefore still enough data are lacking, hence the present findings add more data on the gap of knowledge in this matter. It gives awareness to pregnant women when the need arises when they go to their ANC visits and to the health workers it could guide them for awareness programs, in educating women (pregnant or not pregnant). The potential weakness of this study is: that the site of the study limited the researcher since the study was done at Parirenyatwa Group of Hospitals only. Consequently, it is impossible to rule out potential selection prejudice. The data provided is unable to give us a straight answer on the onset of the disease during pregnancy as many women did not have their high blood pressure checked before pregnancy and during the first weeks of pregnancy. The study is prone to bias as pregnant women who were left out might have different characteristics from those who were involved in the study. Those who were excluded might differ in some ways from those who were included in the research. Also, some would have died because they could not access health services. Records for patients who are already dead have been archived already and this can be hard to access them. Another limitation of this study was his focus on the bivariate analysis of risk factors even though efforts were made through randomization to limit the effect of confounding variables still the study may have some bias hence we recommend a large-scale study involving multivariate analysis of additional risk factors which were not explored in this present study.
Conclusion
There is an increase in the rate of hypertensive disorders of pregnancy in this study, HBP is still a common pregnancy-related disorder in Zimbabwe Risk factors are mainly Previous Family History of HBP, Gravida, parity, ANC, and age where the problem is higher among older women aged ≥ 30 years. Gestational hypertension is the most common finding. Therefore, early detection and treatment for HBP are needed for pregnant mothers aged ≥ 30 years old. National studies and reviews in Zimbabwe are needed to show the association of HBP in pregnancy with other socio-demographic, maternal, and health service-related factors.
Acknowledgments
Approval from the University Research Ethics Committee was granted for the research to be conducted. Approval from the Ethics committee and getting permission from Parirenyatwa Hospital should be done first for the research to be conducted using the protocols approved.
Conflicts of interest
The authors declare they have no competing interests in this research.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
Ethical considerations
This study was approved by the Africa University Research Ethical Committee (AUREC) with ethical code number (AUREC 2616/23).
Code of Ethics
AUREC 2616/23.
Authors’ contributions
L. M, S. O. M and M. T. M. S; designed the works, analysed data, wrote the first draft of the manuscript, and revised the manuscript for its critical contents.
Open access policy
JCHR does not charge readers and their institutions for access to its papers. Full-text downloads of all new and archived papers are free of charge.
Reports from systematic review and meta-analysis including studies from 24 African countries revealed a significantly higher Prevalence of Hypertensive disorders of pregnancy (HDP) in Central and Western Africa; where the burden of HDP in Africa is high, with about one in 10 pregnancies affected (13). In the case of Zimbabwe, our present study indicated socio-demographic characteristics that in majority of the study participants are from urban areas and the majority are in the age range of 20 to 30 years old. In this study, HBP is more common in Urban than rural and a similar finding was found in a study by Raj et al 2018 where a low prevalence was found in rural settings (14). Our findings indicate high prevalence within the age range of 31 to 40 years similar finding was found in a study conducted by Ye et al 2014 in China (15) and Berhe, A. K et al 2018 in Ethiopia (16). The opposite study by Umesawa et al 2017 found a low prevalence (17) our study found a high prevalence of gestational hypertension and preeclampsia Our finding indicates a high prevalence within the age range of 31 to 40 years as reported previously gestational hypertension and preeclampsia increased with gestational age (18). And in this present study this could be associated with unemployment and interrupted ANC which are the most predominant finding associated with these conditions. Umesawa, et al 2017 study similar to our study finding lower education appears to be a modifiable risk factors for HBP. Furthermore similar to their study, our study indicated preexisting hypertension and diabetes mellitus appear to be non-modifiable risk factors (17). The obstetrical factor associated with HBP in pregnancy in this present study is mainly Gestational age at 3rd trimester of pregnancy, Parity 3-4, ANC 1,2. In our study, the prevalence is low among multigravida (25%) while opposite findings were reported in a study conducted in India where The prevalence was high primigravida (54%) compared to multigravida (46%) (19). As the number of fetuses a mother is carrying increases (multiplicity of pregnancy) the risk of high blood pressure in pregnancy increases. Preventing and/or managing high blood pressure in pregnancy lowers the risk of low birth weight and its implications. Another risk factor for high blood pressure in pregnancy includes the fact that pregnant women are facing delays in being attended to during their ANC visits. This is caused by a lack of staff, ambulances, or other vital resources (these include BP machines), which raises the risk of maternal and newborn mortality due to high blood pressure. The prevalence of high blood pressure in pregnant women was 25% (60/240) which is still high when compared to the worldwide Global prevalence, of 2.7% in pregnant women HDP and low when compared to the Tanzania study which found it to be 26.7% (20). Among potential risk factor that significantly leads to HBP found in this study are mainly the family history of high blood pressure and being aged 30 years old and above and this corroborates with studies conducted by (Umesawa et al 2017, and Valerie et al 2021) which reported similar findings (17, 21). It might also be a result of more pregnancies being scheduled as a result of the drop in maternal costs. Many women tend to get pregnant easily since maternal costs are low now, thus a large number of pregnant women were present in the study. Furthermore, the high prevalence of high blood pressure in pregnant women was linked to certain characteristics such as multiplicity of pregnancy. According to previous studies, chronic hypertension, preeclampsia and have been recorded to be 0.29%, and 2.16% in that order (5). Gestational hypertension was 20.7% in 2009 to 44% in 2011 which is still low when compared to this present study which found prevalence for preeclampsia, chronic hypertension, and gestational hypertension at 23%, 17%, and 60% respectively. This showed a high prevalence of high blood pressure in pregnant women stratified by high blood pressure types. The present study reported a high prevalence of Gestational hypertension and pre-eclampsia when compared to a study conducted in India which reported a low prevalence of Gestational hypertension and pre-eclampsia and this disparity could be likely be attributed to differences in study design and study setting which differ among the two studies. However, Similarities with our findings were reported by Valérie et al 2021 in a study conducted in France where chronic hypertension and pre-eclampsia were associated with HBP during pregnancy (21).
Despite previous study has been done in Zimbabwe on the prevalence of HBP in pregnant women (Muti M, 2015) still there has not been a reduction in these morbidity and mortality rates rather there has been an increase in the rates therefore still enough data are lacking, hence the present findings add more data on the gap of knowledge in this matter. It gives awareness to pregnant women when the need arises when they go to their ANC visits and to the health workers it could guide them for awareness programs, in educating women (pregnant or not pregnant). The potential weakness of this study is: that the site of the study limited the researcher since the study was done at Parirenyatwa Group of Hospitals only. Consequently, it is impossible to rule out potential selection prejudice. The data provided is unable to give us a straight answer on the onset of the disease during pregnancy as many women did not have their high blood pressure checked before pregnancy and during the first weeks of pregnancy. The study is prone to bias as pregnant women who were left out might have different characteristics from those who were involved in the study. Those who were excluded might differ in some ways from those who were included in the research. Also, some would have died because they could not access health services. Records for patients who are already dead have been archived already and this can be hard to access them. Another limitation of this study was his focus on the bivariate analysis of risk factors even though efforts were made through randomization to limit the effect of confounding variables still the study may have some bias hence we recommend a large-scale study involving multivariate analysis of additional risk factors which were not explored in this present study.
Conclusion
There is an increase in the rate of hypertensive disorders of pregnancy in this study, HBP is still a common pregnancy-related disorder in Zimbabwe Risk factors are mainly Previous Family History of HBP, Gravida, parity, ANC, and age where the problem is higher among older women aged ≥ 30 years. Gestational hypertension is the most common finding. Therefore, early detection and treatment for HBP are needed for pregnant mothers aged ≥ 30 years old. National studies and reviews in Zimbabwe are needed to show the association of HBP in pregnancy with other socio-demographic, maternal, and health service-related factors.
Acknowledgments
Approval from the University Research Ethics Committee was granted for the research to be conducted. Approval from the Ethics committee and getting permission from Parirenyatwa Hospital should be done first for the research to be conducted using the protocols approved.
Conflicts of interest
The authors declare they have no competing interests in this research.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
Ethical considerations
This study was approved by the Africa University Research Ethical Committee (AUREC) with ethical code number (AUREC 2616/23).
Code of Ethics
AUREC 2616/23.
Authors’ contributions
L. M, S. O. M and M. T. M. S; designed the works, analysed data, wrote the first draft of the manuscript, and revised the manuscript for its critical contents.
Open access policy
JCHR does not charge readers and their institutions for access to its papers. Full-text downloads of all new and archived papers are free of charge.
References
1. Kobashi G. Genetic and environmental factors associated with the development of hypertension in pregnancy. Journal of epidemiology. 2006; 16(1): 1-8.
2. Program NHBPE. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. American journal of obstetrics and gynecology. 2000; 183(1): s1-s22.
3. Tranquilli A, Dekker G, Magee L, et al. The classification, diagnosis, and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Elsevier. 2014; 97-104.
4. Wang L, Ye W, Xiong W, et al. Effects of blood pressure level management on maternal and perinatal outcomes in pregnant women with mild to moderate gestational hypertension. Ginekologia Polska. 2020; 91(3): 137-43.
5. Abalos E, Cuesta C, Carroli G, et al. Pre‐eclampsia, eclampsia, and adverse maternal and perinatal outcomes: a secondary analysis of the World Health Organization Multicountry S survey on M maternal and N newborn H health. BJOG: An International Journal of Obstetrics & Gynaecology. 2014; 121: 14-24.
6. Stuart JJ, Tanz LJ, Missmer SA, et al. Hypertensive disorders of pregnancy and maternal cardiovascular disease risk factor development: an observational cohort study. Annals of internal medicine. 2018; 169(4): 224-32.
7. Noubiap JJ, Bigna JJ, Nyaga UF, et al. The burden of hypertensive disorders of pregnancy in Africa: a systematic review and meta‐analysis. The Journal of Clinical Hypertension. 2019; 21(4): 479-88.
8. Benschop L, Duvekot JJ, van Lennep JER. Future risk of cardiovascular disease risk factors and events in women after a hypertensive disorder of pregnancy. Heart. 2019; 105(16): 1273-8.
9. Tachiweyika E, Gombe N, Shambira G, et al. Determinants of perinatal mortality in Marondera district, Mashonaland East Province of Zimbabwe, 2009: a case-control study. The Pan African Medical Journal. 2011; 8.
10. Muti M, Tshimanga M, Notion GT, et al. Prevalence of pregnancy-induced hypertension and pregnancy outcomes among women seeking maternity services in Harare, Zimbabwe. BMC cardiovascular disorders. 2015; 15: 1-8.
11. Gemechu KS, Assefa N, Mengistie B. Prevalence of hypertensive disorders of pregnancy and pregnancy outcomes in Sub-Saharan Africa: A systematic review and meta-analysis. Womens Health (Lond). 2020; 16: 1745506520973105.
12. Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. The Lancet Global Health. 2014; 2(6): e323-e33.
13. Noubiap JJ, Bigna JJ, Nyaga UF, et al. The burden of hypertensive disorders of pregnancy in Africa: A systematic review and meta-analysis. J Clin Hypertens (Greenwich). 2019; 21(4): 479-88.
14. Raj K, Paul A, Bansal K, et al. Incidence of gestational hypertension among pregnant women in the rural population of District Amritsar-A community-based study. EXECUTIVE EDITOR. 2018; 9(8): 42.
15. Ye C, Ruan Y, Zou L, et al. The 2011 Survey on Hypertensive Disorders of Pregnancy (HDP) in China: Prevalence, Risk Factors, Complications, Pregnancy and Perinatal Outcomes. PLoS ONE. 2014; 9(6).
16. Berhe AK, Kassa GM, Fekadu GA, et al. Prevalence of hypertensive disorders of pregnancy in Ethiopia: a systemic review and meta-analysis. BMC Pregnancy Childbirth. 2018; 18(1): 34.
17. Umesawa M, Kobashi G. Epidemiology of hypertensive disorders in pregnancy: prevalence, risk factors, predictors and prognosis. Hypertens Res. 2017; 40(3): 213-20.
18. Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013; 209(6): 544.e1-.e12.
19. Sengodan SS, Sreeprathi N. Prevalence of hypertensive disorders of pregnancy and its maternal outcome in a tertiary care hospital, Salem, Tamil Nadu, India. International Journal of reproduction, contraception, Obstetrics and Gynecology. 2020; 9(1): 236-40.
20. Larson E, Rabkin M, Mbaruku GM, et al. Missed opportunities to improve the health of postpartum women: high rates of untreated hypertension in rural Tanzania. Maternal and child health journal. 2017; 21: 407-13.
21. Olié V, Moutengou E, Grave C, et al. Prevalence of hypertensive disorders during pregnancy in France (2010-2018): The Nationwide CONCEPTION Study. J Clin Hypertens (Greenwich). 2021; 23(7): 1344-53.
Review: Research |
Subject:
Public Health
Received: 2024/08/21 | Accepted: 2024/10/18 | Published: 2024/10/21
Received: 2024/08/21 | Accepted: 2024/10/18 | Published: 2024/10/21
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution 4.0 International License. |








